CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) is a revolutionary gene editing technology that allows precise modification of DNA sequences in living organisms. It was adapted for genome editing in 2012 by Jennifer Doudna and Emmanuelle Charpentier, who were awarded the 2020 Nobel Prize in Chemistry for their work.
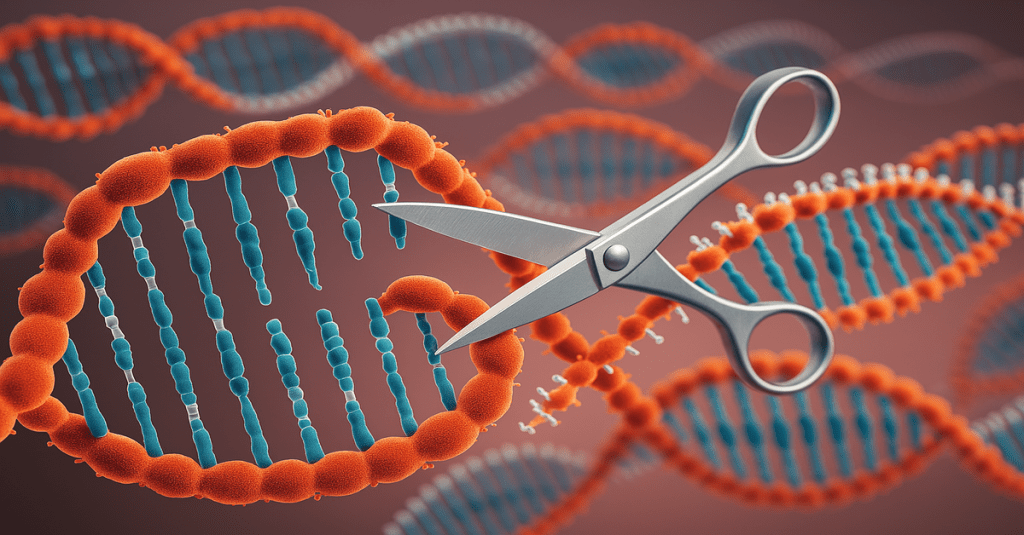
The CRISPR-Cas9 system consists of two main components:
- A guide RNA (gRNA) that locates the target DNA sequence
- The Cas9 enzyme, which acts as “molecular scissors” to cut the DNA at the specified location
This technology enables researchers to add, remove, or alter specific DNA sequences with unprecedented precision and efficiency.
Applications of CRISPR gene editing technology
CRISPR gene editing technology has revolutionized multiple fields from agriculture to medicine. Its applications range from developing disease-resistant crops and engineering microorganisms for biofuel production to advancing medical research and treatments, including gene therapies for genetic disorders, cancer research, and infectious disease studies.
Biotech applications:
- Crop improvement: Developing disease-resistant and climate-resilient plants
- Industrial biotechnology: Engineering microorganisms for biofuel production
- Animal models: Creating genetically modified animals for research
Medical and healthcare applications:
- Gene therapy: Correcting genetic disorders
- Cancer research: Identifying cancer-causing genes and developing targeted therapies
- Infectious disease research: Studying host-pathogen interactions
🧬 FDA-approved therapy
- Casgevy (exa-cel) for sickle cell disease (2023):
- Developed by Vertex Pharmaceuticals and CRISPR Therapeutics, Casgevy is the first CRISPR-based gene therapy approved by the FDA. It received approval on December 8, 2023, for the treatment of sickle cell disease in patients 12 years and older. This therapy uses CRISPR-Cas9 to edit a patient’s hematopoietic stem cells to produce fetal hemoglobin, which helps prevent the sickling of red blood cells.
🧬 Pipeline developments:
- EDIT-101 by Editas Medicine: A therapy for Leber congenital amaurosis 10 (LCA10), a rare genetic eye disorder (Phase 1/2 clinical trials)
- CTX001 by CRISPR Therapeutics: A gene-editing therapy for sickle cell disease and beta-thalassemia.
- EBT-101 by Excision BioTherapeutics: A therapy designed to eliminate HIV proviral DNA (Phase 1/2 clinical trials)
- CTX110 by CRISPR Therapeutics: An allogeneic CAR-T cell therapy for CD19+ B-cell malignancies (Phase 1 clinical trials)
- NTLA-2001 by Intellia Therapeutics and Regeneron: A therapy for transthyretin amyloidosis (Phase 1 clinical trials)
It’s important to note that while CRISPR technology holds immense promise, ethical concerns surrounding its use, particularly in human embryo editing, have led to calls for careful regulation and oversight. The field is rapidly evolving, and new developments are constantly emerging. The approval of Casgevy marks a significant milestone in CRISPR gene therapy, paving the way for future treatments.
References:
- Broad Institute. CRISPR Timeline.
- Gostimskaya, I. (2022). CRISPR–Cas9: A History of Its Discovery and Ethical Considerations of Its Use in Genome Editing. National Center for Biotechnology Information.
- Integrated DNA Technologies. A Short History of CRISPR.
- Encyclopaedia Britannica. Gene Editing.
- Addgene. CRISPR History.
- U.S. Food and Drug Administration. (2023, December 8). FDA Approves First Gene Therapies to Treat Patients with Sickle Cell Disease.
- Vertex Pharmaceuticals. (2023, December 8). Vertex and CRISPR Therapeutics Announce U.S. FDA Approval of CASGEVY™ (exagamglogene autotemcel).
- CRISPR Therapeutics. Pipeline.
- Editas Medicine. Gene Editing Pipeline.
- Excision BioTherapeutics. Technology Pipeline.
Originally published by Romesh Collins on LinkedIn. Access the LinkedIn article here.
